c3h6 lewis dot structure|Lewis Dot Structures : Pilipinas A step-by-step explanation of how to draw the C3H6 Lewis Structure.For this chemical formula there are two different ways to draw the C3H6 Lewis structure. . Time Difference. Pacific Daylight Time is 17 hours behind AEST (Australian Eastern Standard Time) 1:30 am 01:30 in PDT is 6:30 pm 18:30 in Melbourne, Australia. PST to Melbourne call time Best time for a conference call or a meeting is between 4am-6am in PST which corresponds to 10pm-12pm in Melbourne
c3h6 lewis dot structure,Learn how to draw the electron dot structure for C3H6, a chemical formula that can be represented by two different Lewis structures: cyclopropane and propene. Both structures use .
A step-by-step explanation of how to draw the C3H6 Lewis Structure.For this chemical formula there are two different ways to draw the C3H6 Lewis structure. . In this video, we will help you to determine the Lewis structure of all the possible isomers having formula C3H6. .more. In chemistry, isomers are molecules with identical .
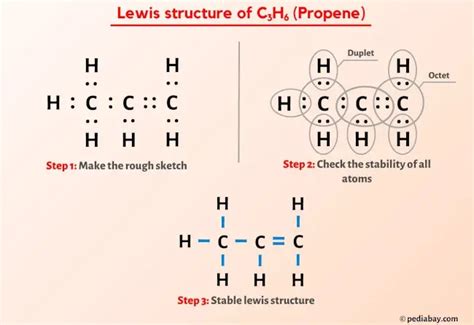
C3H6 (Propene) lewis structure has three Carbon atoms (C) at the center and they are surrounded by the Hydrogen atoms (H). It contains six C-H bonds, one C-C bond and one C=C bond. C 3 H 6 Lewis structure. C 3 H 6 (propene) has three carbon atoms and six hydrogen atoms. In the C 3 H 6 Lewis structure, there is one double bond and one single . The lewis structure of C3H6 has 18 bonding electrons and zero non-bonding electrons. The drawing of the Propene (C3H6) lewis structure is an easy and simple process. .
Lewis Structures. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and .
To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one .
Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess .c3h6 lewis dot structure Lewis Dot Structures In the periodic table, carbon lies in group 14, and hydrogen lies in group 1.. Hence, carbon has four valence electrons and hydrogen has one valence electron.. Since C 6 H 6 has six carbon atoms and six hydrogen . A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . To draw the Lewis structure of an atom, write the .

A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in .Learn about the formula and structure of propylene, a hydrocarbon with double bond, and explore its 3D model with Jmol applet.Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. You learn new concepts such as bond length, bond energy, bond order, covalent bond .
The 2D chemical structure image of CYCLOPROPANE is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of CYCLOPROPANE are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with . 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One.Question: Select the correct Lewis Dot Structure for C3H6. Figure A Figure B Н Figure C H. "Croco.C..H .H •H C.. Cool ..H •C:62 C:::: co H。
Made with Explain Everything Here, I have explained simple steps to draw the lewis dot structure of Acetone (along with images). So let’s dive right into it! Lewis structure of Acetone (also known as Propanone or C3H6O) contains three Carbon atoms (C) in a row which have an Oxygen atom (O) attached to a central Carbon atom (C) forming a double bond. The outer Carbon .

Examine the Lewis dot structure of propene, C,H, and answer the following questions, H- C- C= HHH How many groups of electrons are around carbon atom B in propene? Are the groups of electrons around carbon atom B in propene . Infact, I’ve also given the step-by-step images for drawing the lewis dot structure of C2H4Cl2 molecule. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of C2H4Cl2 (1, 2-dichloroethane) contains a single bond between the two Carbon (C) atoms, Carbon-Hydrogen atoms and Carbon-Chlorine atoms. Compute formal charges for atoms in any Lewis structure; Use formal charges to identify the most reasonable Lewis structure for a given molecule; Identify the oxidation states of atoms in Lewis structures; 3.3.0: Bond Types. 3.3.0.0: Bond Types (Problems) 3.3.1: Lewis Dot Diagrams. 3.3.1.0: Lewis Dot Diagrams (Problems) 3.3.2: Lewis Structures . We used only condensed structural formulas in Table \(\PageIndex{1}\). Thus, CH 2 =CH 2 stands for. The double bond is shared by the two carbons and does not involve the hydrogen atoms, although the condensed formula does not make this point obvious. Note that the molecular formula for ethene is C 2 H 4, whereas that for ethane is C 2 H 6.
Lewis Dot Structures The lewis structure of C3H8 has 20 bonding electrons and zero non-bonding electrons. The drawing of the Propane lewis structure is an easy and simple process. Let’s see how to do it. Follow some steps for drawing the lewis dot structure for C3H8. 1. Count total valence electron in C3H8
Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule.Lewis Dot Structure. Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron. A step-by-step explanation of how to draw the CH3OH Lewis Structure.When you see a carbon with an OH attached (like CH3OH, C2H5OH, etc.) that means that he O.
c3h6 lewis dot structure|Lewis Dot Structures
PH0 · Lewis Structure of C3H6 (Propene) (In 3 Simple Steps)
PH1 · Lewis Dot Structures
PH2 · Chapter 5.3: Lewis Structures
PH3 · C3H6 Lewis structure, Hybridization, Molecular geometry, Polarity
PH4 · C3H6 Lewis structure
PH5 · C3H6 Lewis Structure: How to Draw the Lewis Structure for C3H6.
PH6 · C3H6 Lewis Structure: How to Draw the Lewis Structure for C3H6
PH7 · C3H6 Lewis Structure
PH8 · C3H6 (Propene) Lewis Structure in 3 Steps (With
PH9 · 9.3: Drawing Lewis Structures
PH10 · 7.3 Lewis Symbols and Structures